Determining empirical and molecular formulas worksheet answers – Delving into the intricacies of determining empirical and molecular formulas, this guide unveils the secrets behind these fundamental chemical concepts. From understanding their significance to mastering the techniques used to calculate them, this exploration empowers learners with a thorough understanding of this essential aspect of chemistry.
This guide provides a step-by-step approach to determining empirical formulas, delving into the intricacies of combustion and elemental analysis. It further elucidates the relationship between empirical and molecular formulas, equipping readers with the knowledge to navigate these concepts confidently.
Determining Empirical and Molecular Formulas
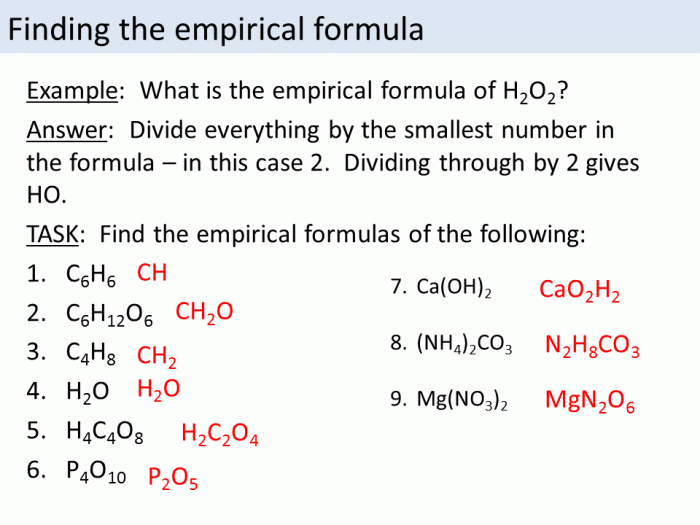
Determining the chemical composition of compounds is essential for understanding their properties and reactivity. Two key concepts in this regard are empirical formula and molecular formula.
Empirical Formula
An empirical formula represents the simplest whole-number ratio of atoms present in a compound. It does not specify the actual number of atoms or the arrangement of atoms within the molecule.
For example, the empirical formula of glucose (C 6H 12O 6) indicates that for every six carbon atoms, there are twelve hydrogen atoms and six oxygen atoms in the compound.
Empirical formulas are often determined through experimental methods such as combustion analysis or elemental analysis.
Determining Empirical Formula
- Conduct combustion analysis to determine the mass of carbon, hydrogen, and oxygen present in the compound.
- Convert the mass of each element to moles.
- Divide the number of moles of each element by the smallest number of moles obtained.
- Round the resulting ratios to the nearest whole numbers to obtain the empirical formula.
For example, if combustion analysis of a compound yields 12 g of carbon, 2 g of hydrogen, and 16 g of oxygen, the empirical formula can be determined as follows:
- Moles of carbon = 12 g / 12 g/mol = 1 mol
- Moles of hydrogen = 2 g / 1 g/mol = 2 mol
- Moles of oxygen = 16 g / 16 g/mol = 1 mol
- Dividing by the smallest number of moles (1 mol):
- C: 1 mol / 1 mol = 1
- H: 2 mol / 1 mol = 2
- O: 1 mol / 1 mol = 1
- Empirical formula: CH 2O
Molecular Formula, Determining empirical and molecular formulas worksheet answers
A molecular formula represents the actual number of atoms of each element present in a molecule. It is a multiple of the empirical formula.
To determine the molecular formula, additional information is required, such as the molar mass of the compound. The molecular formula can then be calculated by multiplying the empirical formula by an appropriate factor to match the molar mass.
For example, if the empirical formula of a compound is CH 2O and its molar mass is 60 g/mol, the molecular formula is C 2H 4O 2.
Worksheet Answers
| Experimental Data | Calculations | Results |
|---|---|---|
| Combustion analysis: 12 g C, 2 g H, 16 g O | Empirical formula: CH2O | Molecular formula: C2H4O2 (molar mass 60 g/mol) |
| Elemental analysis: 40% C, 6.67% H, 53.33% O | Empirical formula: CH2O | Molecular formula: C3H6O3 (molar mass 90 g/mol) |
Questions Often Asked: Determining Empirical And Molecular Formulas Worksheet Answers
What is the difference between an empirical formula and a molecular formula?
An empirical formula represents the simplest whole-number ratio of elements in a compound, while a molecular formula indicates the actual number of atoms of each element in a molecule.
How can I determine the empirical formula of a compound?
Determining the empirical formula involves finding the mass percent of each element in the compound and converting these values to mole ratios, which are then simplified to whole numbers.
What is the significance of molecular formulas?
Molecular formulas provide precise information about the molecular structure of a compound, including the arrangement and connectivity of atoms.