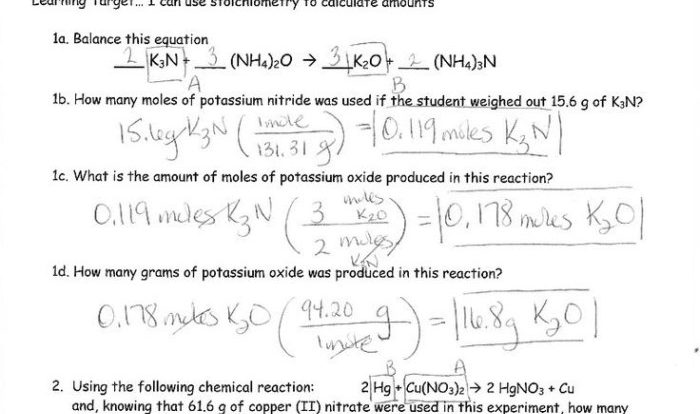
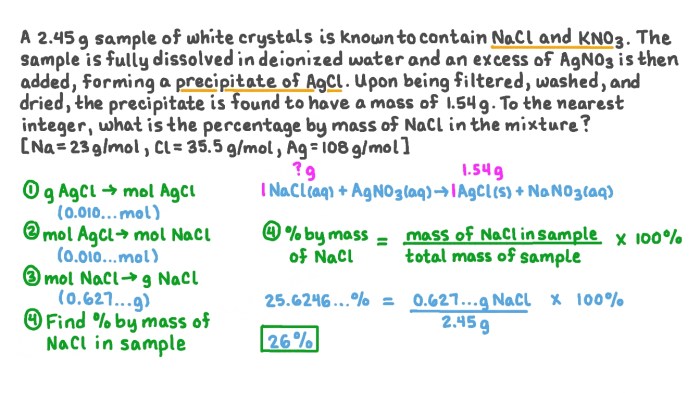
Elemental sulfur can exist as molecules with the formula S8, revealing a fascinating world of chemical bonding and molecular structure. This unique form of sulfur exhibits remarkable properties and finds applications in diverse fields, ranging from agriculture to pharmaceuticals. Join us as we delve into the intriguing characteristics, reactions, and environmental impact of S8.
The formation and reactivity of S8 molecules hold the key to understanding their stability and behavior in different environments. We will explore the intricate chemical reactions involving S8, including oxidation and reduction, and examine how these reactions influence its reactivity and stability.
Elemental Sulfur (S8) Overview
Elemental sulfur, represented by the formula S8, is an allotrope of sulfur that exhibits unique chemical and physical properties. S8 molecules consist of eight sulfur atoms arranged in a cyclic structure, forming a puckered ring.
These S8 molecules possess distinct characteristics that set them apart from other forms of sulfur. The cyclic structure results in a relatively low melting point and high vapor pressure, making S8 readily volatile. Additionally, S8 molecules are insoluble in water but soluble in nonpolar organic solvents.
Formation and Reactions of S8: Elemental Sulfur Can Exist As Molecules With The Formula S8
The formation of S8 molecules occurs through a process called the “S8 ring-closing reaction.” In this reaction, eight sulfur atoms combine to form a cyclic structure, releasing energy in the form of heat.
S8 molecules can undergo various chemical reactions, including oxidation and reduction. Upon oxidation, S8 is converted to sulfur dioxide (SO2) or sulfur trioxide (SO3), depending on the reaction conditions. Conversely, reduction of S8 can produce hydrogen sulfide (H2S) or other sulfur-containing compounds.
The reactivity of S8 varies depending on the environment. In the presence of oxygen, S8 is readily oxidized, while in reducing environments, it remains relatively stable.
Applications of Elemental Sulfur

- Agriculture:S8 is used as a soil amendment to reduce soil pH and provide essential nutrients for plants.
- Pharmaceuticals:S8 is employed in the production of various medications, including antibiotics, antifungals, and anti-inflammatory drugs.
- Energy:S8 is utilized in the extraction and refining of petroleum and natural gas.
- Other Applications:S8 finds use in the manufacturing of rubber, dyes, and matches.
Environmental Impact of S8
Elemental sulfur emissions can have adverse effects on the environment. Combustion of fossil fuels and industrial processes release S8 into the atmosphere, contributing to air pollution.
In water bodies, S8 can impact aquatic ecosystems by altering pH levels and affecting the availability of oxygen. Additionally, S8 can be toxic to certain organisms, particularly at high concentrations.
Mitigation strategies for reducing the environmental impact of S8 include controlling emissions from industrial sources, promoting the use of alternative fuels, and implementing wastewater treatment processes.
Comparisons with Other Sulfur Forms
Elemental sulfur exists in various allotropes, including S2, S6, and S7, each with distinct properties and reactivity.
S2 molecules are linear and highly reactive, while S6 and S7 molecules adopt more complex cyclic structures. The differences in molecular structures result in variations in melting points, solubilities, and chemical reactivity.
The choice of sulfur allotrope for specific applications depends on the desired properties and the chemical environment in which it will be used.
Helpful Answers
What is the significance of S8 molecules?
S8 molecules are unique due to their cyclic structure and distinct properties, which enable their use in various industrial and commercial applications.
How does S8 react in different environments?
S8 exhibits varying reactivity depending on the environment. It undergoes oxidation and reduction reactions, and its stability is influenced by factors such as temperature and the presence of other chemical species.
What are the environmental implications of S8?
S8 emissions can impact air and water quality, and its potential toxicity requires careful consideration. Mitigation strategies are essential to minimize the environmental impact of S8.