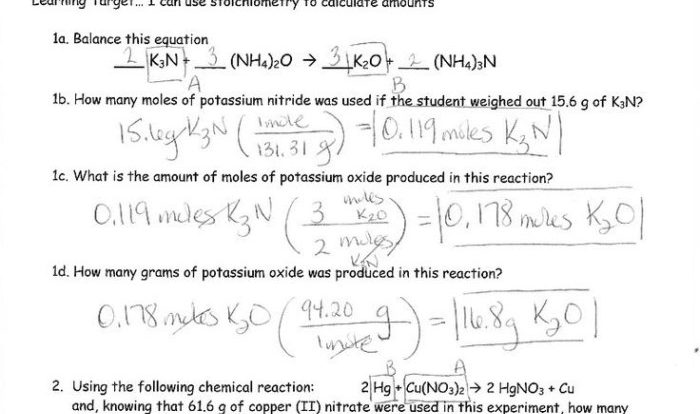
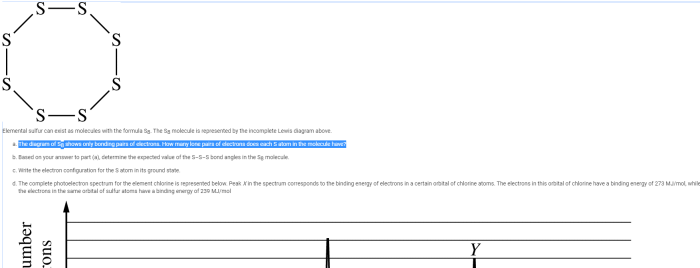
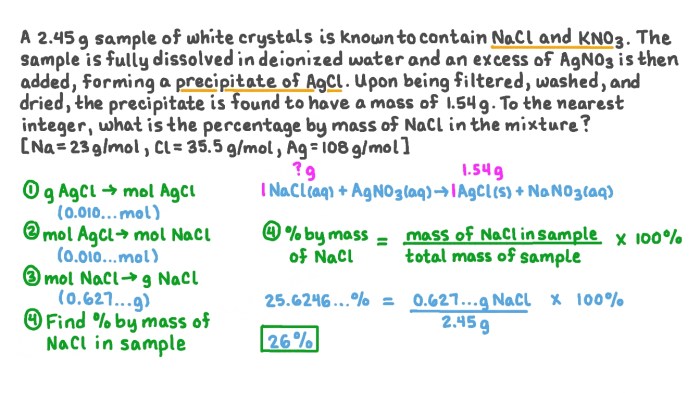
The reaction shown is of what type? This captivating inquiry unveils the intricate tapestry of chemical reactions, inviting us to explore the diverse mechanisms, factors, and applications that govern these fundamental processes. Join us as we embark on a journey into the realm of chemical transformations, deciphering the language of reactions and unlocking the secrets they hold.
From the interplay of reactants and products to the influence of catalysts and energy changes, we delve into the intricate mechanisms that drive chemical reactions. We uncover the factors that determine the reaction type, the rates at which they proceed, and the equilibrium positions they establish.
Reaction Type Identification
Chemical reactions involve changes in the chemical composition of substances, resulting in the formation of new substances. These reactions can be classified into various types based on their characteristics. Understanding reaction types is crucial in chemistry, as it provides insights into the behavior of substances and helps predict the outcome of reactions.
Factors Determining Reaction Type
Several factors influence the type of chemical reaction that occurs, including:
- Nature of the reactants
- Reaction conditions (temperature, pressure, presence of a catalyst)
- Electronic structure of the reactants
- Thermodynamic and kinetic factors
Reaction Mechanisms
Reaction mechanisms provide detailed explanations of how chemical reactions occur, elucidating the step-by-step transformations of reactants into products.
These mechanisms involve the breaking and formation of chemical bonds, often mediated by the participation of catalysts or the transfer of energy.
Role of Catalysts
Catalysts are substances that increase the rate of a reaction without being consumed themselves. They facilitate reactions by providing alternative pathways with lower activation energies, allowing reactions to proceed more rapidly at lower temperatures or with reduced energy input.
Energy Changes in Reactions, The reaction shown is of what type
Chemical reactions involve energy changes. Exothermic reactions release energy in the form of heat or light, while endothermic reactions require an input of energy to proceed. The activation energy is the minimum amount of energy required to initiate a reaction, and it can be lowered by the presence of catalysts.
Reaction Rates
The rate of a reaction refers to the speed at which reactants are converted into products. Understanding reaction rates is crucial in various fields, including chemistry, biology, and engineering. Several factors influence the rate of a reaction, including the concentration of reactants, temperature, surface area, and the presence of a catalyst.
Concentration of Reactants
The concentration of reactants plays a significant role in determining the reaction rate. Generally, the higher the concentration of reactants, the faster the reaction proceeds. This is because a higher concentration means more reactant particles are available to collide with each other, increasing the probability of a successful reaction.
Temperature
Temperature is another crucial factor that affects reaction rates. As temperature increases, the average kinetic energy of the reactants increases, leading to more frequent and energetic collisions. Consequently, the reaction rate increases with temperature.
Surface Area
For reactions involving solids, the surface area of the reactants can significantly impact the reaction rate. A larger surface area provides more contact points for the reactants to interact, resulting in a faster reaction.
Activation Energy
Activation energy refers to the minimum amount of energy required for a reaction to occur. Reactants must overcome this energy barrier to reach the transition state, where the reaction can proceed. The higher the activation energy, the slower the reaction rate.
Catalysts
Catalysts are substances that can accelerate the rate of a reaction without being consumed in the process. They provide an alternative pathway for the reaction to occur, lowering the activation energy and making the reaction proceed faster.
Calculating Reaction Rates
Reaction rates can be calculated using various methods, including the initial rate method, the integrated rate law method, and the half-life method. The choice of method depends on the availability of data and the order of the reaction.
- Initial Rate Method:Measures the initial rate of the reaction, which is the rate at the beginning of the reaction when the concentrations of reactants are highest.
- Integrated Rate Law Method:Uses the integrated rate law to determine the rate constant and the order of the reaction.
- Half-Life Method:Determines the half-life of the reaction, which is the time required for the concentration of a reactant to decrease by half.
Reaction Equilibrium: The Reaction Shown Is Of What Type
Chemical equilibrium is a state in which the concentrations of the reactants and products of a chemical reaction do not change over time. This means that the forward and reverse reactions are occurring at the same rate.
The equilibrium position of a reaction is the point at which the concentrations of the reactants and products are equal. This position is determined by several factors, including the temperature, pressure, and the concentration of the reactants and products.
Factors Affecting Equilibrium Position
- Temperature:Increasing the temperature of a reaction shifts the equilibrium position to the side of the products. This is because the higher temperature provides more energy for the reaction to occur.
- Pressure:Increasing the pressure of a reaction shifts the equilibrium position to the side of the reactants. This is because the higher pressure forces the reactants to come together and react.
- Concentration of reactants and products:Increasing the concentration of the reactants shifts the equilibrium position to the side of the products. This is because the higher concentration of reactants increases the likelihood of them colliding and reacting.
Calculating Equilibrium Constants
The equilibrium constant is a measure of the extent to which a reaction proceeds to completion. It is calculated by dividing the concentration of the products by the concentration of the reactants at equilibrium.
For example, the equilibrium constant for the reaction:
A + B <=> C
is:
K = [C]/([A][B])
where [A], [B], and [C] are the concentrations of the reactants and products at equilibrium.
Applications of Reaction Analysis
Reaction analysis plays a crucial role in various scientific disciplines and industrial applications. It provides insights into the behavior of chemical reactions, enabling scientists and engineers to design new products and processes, address environmental challenges, and advance our understanding of the world around us.
In the realm of product development, reaction analysis is instrumental in optimizing existing products and creating innovative ones. By studying the reaction mechanisms and kinetics, researchers can identify ways to improve product performance, reduce costs, and enhance safety. For instance, in the pharmaceutical industry, reaction analysis is used to design new drugs with improved efficacy and reduced side effects.
Environmental Problem Solving
Reaction analysis also serves as a powerful tool for solving environmental problems. It helps scientists understand the chemical processes underlying environmental issues, such as air pollution, water contamination, and climate change. By analyzing the reactions involved in these processes, researchers can develop strategies to mitigate their impact and protect the environment.
For example, reaction analysis has been used to design catalytic converters that reduce harmful emissions from vehicles and to develop technologies for cleaning up oil spills.
FAQ
What are the different types of chemical reactions?
Chemical reactions can be classified into various types, including synthesis, decomposition, single displacement, double displacement, and combustion.
What factors determine the type of reaction that occurs?
The type of reaction that occurs depends on the nature of the reactants, their concentrations, temperature, and the presence of a catalyst.
How can we calculate reaction rates?
Reaction rates can be calculated using various methods, including the initial rate method, the integrated rate law method, and the half-life method.
What is chemical equilibrium?
Chemical equilibrium is a state in which the forward and reverse reactions of a chemical reaction occur at equal rates, resulting in no net change in the concentrations of the reactants and products.
How can reaction analysis be used to solve environmental problems?
Reaction analysis can be used to identify and mitigate environmental pollutants, develop sustainable processes, and optimize energy efficiency.